does autoclaving destroy endotoxins|Endotoxins: A critical concern for critical : manufacture The bacterial endotoxin test (BET) is a relatively straight - . • Moist heat—conventional autoclaving will not destroy endotoxin. However, the combination of . the process does not neces . User friendly programmable Controller with options of pre-vacuum, free-steaming and post .
{plog:ftitle_list}
Frete grátis no dia Compre Autoclave Para Manicure parcelado sem juros! Saiba mais sobre .
Standard laboratory autoclaving procedures have little or no effect on endotoxin levels. Moreover, if the autoclave has previously been used for bacteria, the glassware will become extensively . It is notable that autoclaving silk proteins is more easily scalable and likely less expensive than the endotoxin removal method developed by Hedhammar et al., as autoclaving does not require any .
The bacterial endotoxin test (BET) is a relatively straight - . • Moist heat—conventional autoclaving will not destroy endotoxin. However, the combination of . the process does not neces .Autoclaving can sterilize the surface of glassware, however, endotoxins that cling to sides tend to be too heat-resistant to be removed. They need to be exposed to high dry heat temperatures for an extended period of time (i.e. 250 °C for 30 minutes. It is believed that autoclaving and boiling doesn’t destroy all the endotoxin present.
Since endotoxin is highly heat-stable, standard autoclaving will not destroy endotoxin. . To ensure that endotoxin does not further compromise an already difficult literature or our understanding of biocompatibility and more importantly to prevent the serious health complications of endotoxin contamination, it is the responsibility of every .during washing. While standard laboratory autoclaving procedures will sterilize glassware, they have little if any effect on endotoxin levels due to endotoxin’s high heat stability. Subjecting glassware to 250°C for more than 30 minutes or 180°C for three hours is recommended to destroy any contaminating endotoxin24. This has the added .Laboratory Testing Services for Medical Devices in Rhode IslandTraditional autoclaving does not destroy endotoxins. However, when combined with hydrogen peroxide and pressure, it is effective. Dry heat: Endotoxins are destroyed by exposure to high temperature. Open in a new tab. 4. Sterilization of Ophthalmic Nanopharmaceuticals.
Fighting endotoxins requires a multifaceted, industry- and application-driven approach to cleanroom contamination control. Standard autoclaving procedures used in laboratories do not effectively reduce endotoxin levels, and unused endotoxin-free plasticware is often recommended in place of reusable glassware.
Fighting endotoxins requires a multifaceted, industry- and application-driven approach to cleanroom contamination control. Standard autoclaving procedures used in laboratories do not effectively reduce endotoxin levels, and unused endotoxin-free plasticware is often recommended in place of reusable glassware.
Note: Autoclaving sterilizes glassware/tools but does not depyrogenate it. 6. To depyrogenate plastic tubing, stir bars and other tools which cannot tolerate baking, flush with Cavicide, then thoroughly rinse with sterile, endotoxin-free water. Running acetonitrile through an HPLC system following sterile, depyrogenated water is alsoDoes autoclaving remove endotoxins? No, autoclaving does not remove endotoxins, as they are heat-stable. Special depyrogenation techniques are required to eliminate them. . Antibiotics do not treat endotoxins directly, but they can kill Gram-negative bacteria, reducing the source of endotoxins. However, the breakdown of bacteria can initially .Standard laboratory autoclaving procedures have little or no effect on endotoxin levels. Moreover, if the autoclave has previously been used for bacteria, the glassware will become extensively contaminated with endotoxin molecules. Heating glassware at 180°C overnight is recommended to destroy any attached endotoxin molecules.Standard laboratory autoclaving procedures have little or no effect on endotoxin levels. Moreover, if the autoclave has previously been used for bacteria, the glassware will become extensively contaminated with endotoxin molecules. Heating glassware at 180°C overnight is recommended to destroy any attached endotoxin molecules.
Does autoclaving get rid of endotoxin? Autoclaving can sterilize the surface of glassware, however, endotoxins that cling to sides tend to be too heat-resistant to be removed. . It is believed that autoclaving and boiling doesn’t destroy all the endotoxin present. That is to say depyrogenation is more difficult than sterilization.Endotoxins are small bacterially-derived hydrophobic lipopolysaccharide (LPS) molecules that can easily contaminate labware and whose presence can significantly impact both in vitro and in vivo experiments.Their presence is detected by the limulus amebocyte lysate which can detect down to 0.01 endotoxin units (EU)/mL.Endotoxins are approximately 10 kDa in size, but readily form .“Bacterial endotoxins are not destroyed by the ETO process.”10 The document provides no source to support this claim. Such unsupported statements are not unique in discussions of sterilization and endotoxin. Numerous reports, including recent work, claim that “autoclaving cannot eliminate endotoxin”11 or “endotoxin is not reliablyEndotoxins – all: Pertussis toxins – all: Anatoxin A: Enterobacteriaciae toxins – all: Phalloidin: . When using sodium hypochlorite and / or sodium hydroxide to destroy toxins, the procedure(s) must be performed in a laboratory fume hood or a biological safety cabinet. . destroy toxins by autoclaving them using the procedure outlined .
Sterilization refers to the destruction of living cells. However, the process does not necessarily destroy microbial by-products and toxins. Endotoxin is one toxin that is extremely heat stable and is not destroyed by standard sterilization cycles (e.g., autoclaving).Characteristics of Bacterial Endotoxin . It has been reported that 650 C for 1 minute or 180 C for 4 hours, likewise, will destroy pyrogens. Studies by Tsuji et al, published in 1978, have shown .Depyrogenation refers to the removal of pyrogens from solutions, most commonly from injectable pharmaceuticals.. A pyrogen is defined as any substance that can cause a fever. Bacterial pyrogens include endotoxins and exotoxins, although many pyrogens are endogenous to the host.Endotoxins include lipopolysaccharide (LPS) molecules found as part of the cell wall of .
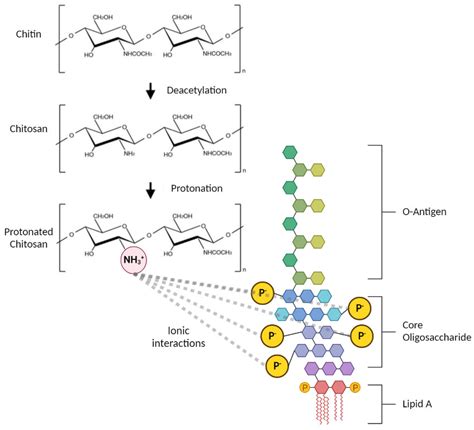
In one experiment, the authors described test results—“after 90 min, endotoxins treated with a 50% steam saturation ratio were not inactivated (LRV [Log Reduction Value] of 1.37 ± 0.30)”—in which detectable endotoxin was reduced by 1.37 logs (~95%) accompanied by a statement that endotoxin was not inactivated, as the result did not . They are padded to prevent rupturing but the disadvantage for that is that solution can get absorbed in between. Since the size endotoxins can vary widely, ultrafilters can be used and this method is called depyrogenation. However, filters are limited to the size of endotoxins they can remove. Smaller endotoxins can manage to cross through the .Autoclaving kills microorganisms but does not remove endotoxin, which can interfere with cell and tissue culturing and provokes immune system activation or even endotoxin shock in animals (Vetten .
GNB endotoxin is a high molecular weight complex that contains lipopolysaccharide (LPS), protein, and phospholipid originating from the outer membrane of Gram-negative bacteria. . since GNB endotoxins are thermostable in the presence of moist heat and are not significantly destroyed by conventional autoclaving processes . Moreover, another .Endotoxin-spiked experimental lots of an iodinated nonionic x-ray contrast solution and a citrated saline solution were studied to determine the effectiveness of heating and pH on reduction of endotoxin levels. Inactivation of the Escherichia coli endotoxins in both 50 mM citrate saline and the nonionic contrast solution was accomplished when the medium was heated (80–90 °C) at .
Removal Of Bacterial Endotoxins
Endotoxins: A critical concern for critical
I get endotoxins in my antibodies that are purifed on Protein A column in a chromatography system. The sample are tested before introduced into the system and are negative. I have tried to wash .
Autoclaving is a type of sterilization that uses high-pressure cooking and is usually operated at 5–15 . Irradiation is a process where feed is exposed to ionizing radiation, which causes chemical changes that destroy DNA and reduce microbial contamination of feeds (Hanis et al., 1988). The dose of irradiation affects the level of microbial .

Out of all EtO alternatives, steam sterilization offers the most benefits. Not only is it just as effective as EtO , but it provides fast and efficient sterilization for metal instruments, .
does autoclaving destroy endotoxins|Endotoxins: A critical concern for critical